Dedicated enquiry hotline 9 am until 6 pm weekdays
Transportation of
Investigational products
Reliable
-All quality control practices employed by Saroute conform to good clinical practice (GCP) and good distribution practice (GDP).
-Our medical logistics professionals, all of whom have completed a specialized training program, will attend to your transportation needs, from start to finish.
Experienced
-Saroute has over 10 years of experience and expertise in the transportation of Investigational products
-We have extensive experience with investigator-initiated clinical trials, large clinical trials, and clinical tests.
-We also transport clinical trial products for use in the field of regenerative medicine.
Flexible
-We offer solutions to a range of challenges relating to temperature control, specified delivery time, and mode of delivery.
-We are also able to provide all necessary packaging, and pack specimens on-site.
TOP > Transportation of Investigational products
We will propose a solution tailored to
the trial size and the transportation conditions.
Since the amendment of the Ministerial Order on Good Clinical Practice in 2008 opened the way for third-party contractors to deliver drugs for clinical trials, Saroute has accumulated a large volume of experience in the delivery of Investigational products for use in investigator-initiated and sponsor-initiated clinical trials. We can propose a tailored solution that is appropriate to the size of your trial and the conditions for transportation of the drug, product, or device being trialed.

Benefits
 No more buying and storing shipping materials means
No more buying and storing shipping materials means
labor savings and reduced costs.
Storing and keeping tabs on shipping boxes, gel-packs, cushioning material, temperature data loggers and other supplies was a major headache.
The transportation of Investigational products necessitates strict temperature control. Procuring and organizing the shipping boxes, coolants, and other supplies required to maintain shipments at a constant temperature tends to put a drain on storage space and personnel resources.


Limited resources can now be used for their intended purposes.
When you entrust Saroute with your transportation needs, you are released from the need to purchase, store, and manage packaging, and can free up the storage, personnel, and financial resources you had been devoting to shipping.
 Saroute Employs Trained Staff for a Delivery Service
Saroute Employs Trained Staff for a Delivery Service
You Can Trust
The client was worried that their trial drugs would not be shipped properly.
It is only natural to feel uneasy at the thought of trial drugs, which require strict temperature control, being transported alongside the whole load of other cargo. The transportation of these drugs requires solid expertise gained through experience and specialized knowledge.

You can trust Saroute’s trained staff.
At Saroute, only staff who have undergone an appropriate training course can be involved in the transportation of Investigational products. You can entrust us to manage and handle Investigational products in transit as well as procure the appropriate packaging and pack the specimens, and take care of the paperwork and verification.
 We provide packaging to suit your requirements to take away
We provide packaging to suit your requirements to take away
your temperature control worries.
The client worried that the temperature-controlled containers they bought might not be able to maintain the required temperature during transit.
As the ambient temperature during transit varies, there is no guarantee that a container that can maintain the required temperature for a given length of time indoors will be able to withstand the punishing conditions of transit.


Saroute will find a container and transport route just right for your requirements!
The containers used by Saroute have undergone temperature stability testing and can be combined with a logger as necessary. We will plan the transportation route (and journey time) in accordance with the cooling time of the shipping box.
Applications
Investigator-Initiated Clinical Trials

- 【Clients】
- University hospitals, medical institutions, contract research organizations (CROs)
- 【Cargo】
- Drugs, products, and devices that are the subject of clinical trials
Sponsor-Initiated Clinical Trials

- 【Clients】
- Pharmaceutical companies, CROs, logistics and warehousing companies
- 【Cargo】
- Drugs, products, and devices that are the subject of clinical trials
What we Transport
Saroute is able to transport
a wide variety of items, including:
Trial drugs, trial products (including cell stocks and intermediary products), and devices that are the subject of clinical trials We can also collect the aforementioned articles and transport them to other institutions.
Saroute undertakes all manner of assignments, from the collection and transportation of trial drugs to the delivery of these drugs to multiple institutions.

Services
Saroute offers
a nationwide service.
In addition to the locations above, transportation staff who have undergone specialized training are posted in other locations as well. Saroute’s nationwide network extends all the way from Hokkaido to Okinawa.
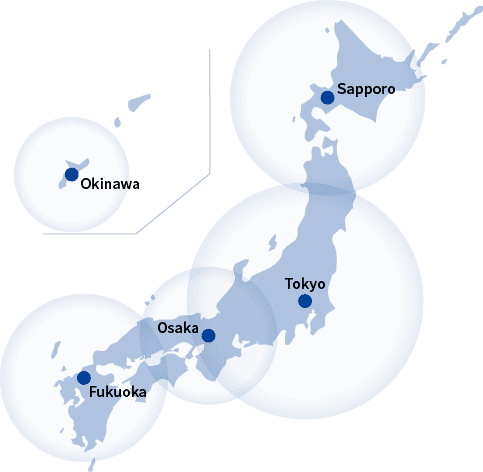
Saroute’s quality control ensures a high degree of safety and reliability.
Saroute has built a rigorous system of quality controls that govern its medical logistics services.
In each part of the network, our professional quality control staff work as a team to ensure safety and peace of mind.
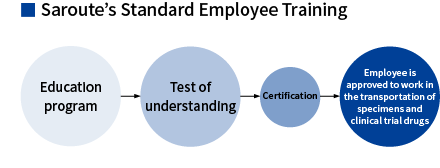
Comprehensive Training for Professional Staff
At Saroute, all staff engaged in providing medical logistics services are required to undergo basic training once a year. Employees are trained in a range of areas including Saroute’s mission, rules in the health sector, Saroute’s standard operating procedure, and compliance. Finally, employees sit a test to check their level of understanding. Individual employees are also trained on procedure and business instructions related to their responsibilities, as necessary.
Whether it’s a one-off order or a large or ongoing project, Saroute provides a service tailored to your needs.
From solutions for the individual specimen transportation requirements of the research community to logistics support for major clinical trials and research conducted at multiple facilities (formulation of transportation networks for clinical specimens), we handle it all. Feel free to contact us with your enquiries.

Whether it’s a one-off order or a large or ongoing project, Saroute provides a service tailored to your needs.
Saroute offers a wide range of services: we handle everything from one-off shipments to the formulation of transportation systems for major projects, irrespective of the number and physical size of the trial drugs involved and the terms of transport.

Large Projects and Tailored Solutions
Saroute can formulate logistics solutions for regenerative medicine/clinical phases
In addition to designing and implementing logistics solutions for Investigational products that are subject to strict control conditions, Saroute also has experience in formulating of domestic cryogenic logistics networks for the transportation of trial products manufactured overseas. We can provide assistance right from the logistics design stage.

We have a solution for your budget and
requirements!
Whether you want to create a bespoke logistics system or are wondering how much it would cost to transport specimens under a given set of conditions, Saroute can offer a solution that fits your budget and transportation requirements.

Examples of Actual Services Provided
-We stored clinical trial products manufactured overseas temporarily in Japan, for delivery on a specified date and time (in conjunction with Saroute affiliate Phicell).
-We entered into an agreement with the medical institution performing a trial so that we could deliver trial drugs to patients’ homes, at the request of the trial sponsor.
-We prepared a written protocol for the shipping of test drugs after interviewing related parties.
Do you need help arranging transport of Biological samples or Investigational products?
Are you facing other logistical challenges in the medical/biotech sector?
Our knowledgeable team of specialists is here to help.
Feel free to ask about our services.
(You can also set up an online meeting to discuss your requirements.)
When formulating a transportation plan, Saroute considers temperature, time, and other variables as well.
So that we can respond to various transportation conditions and requests, and any irregular situations that suddenly manifest themselves, Saroute takes a flexible approach to transport arrangements and maintains a network that leaves nothing to chance.

We Provide Temperature Control at All Ranges
Saroute can provide packaging suitable for various temperatures ranges, including 2 to 8℃, 15 to 25℃, 1 to 30℃, -20℃ or below, -70℃ or below, -150℃ or below, and 37°C±1°C.
A temperature data logger can be used to record the temperature of your shipment in transit. We can also provide you with a log of temperature during transit in PDF format.

Expedited/Same-Day Shipping Available Within
Japan Specify your preferred delivery time!
During transit, the conditions of the cargo are monitored. In the event that the shipment is delayed due to unforeseen circumstances, we have alternative means of transport at the ready at all times in order to make up lost time. Our network enables expedited or same-day delivery within Japan. You can also specify the delivery time.

You Can Also Use Your Own Packaging.
We ensure that the packaging is appropriate for the physical profile of the test drug package or the test device being shipped and the temperature requirements, and will even design and assemble packaging if required. You are also welcome to provide your own packaging.

Examples of Actual Services Provided
-We transported a test drug from Tokyo to Sapporo in a cold box that maintained temperature between 2 and 8°C for quality testing.
-After agreeing a date and time of delivery with the recipient medical institution, we procured packaging in time to meet the client’s delivery schedule, picked up the shipment from the warehouse that was storing the trial drug, and delivered it.
-We shipped trial drugs that are classified as poisons.
Do you need help arranging transport of Biological samples or Investigational products?
Are you facing other logistical challenges in the medical/biotech sector?
Our knowledgeable team of specialists is here to help.
Feel free to ask about our services.
(You can also set up an online meeting to discuss your requirements.)
Your GCP and GDP-Compliant Medical Courier
Saroute offers a range of safe and reliable delivery services that comply with all regulations and guidelines, backed up by 25 years of experience and expertise in medical logistics.
Saroute is accredited to ISO 9001:2015*
*Accredited for: Operations and transportation relating to medical and biological logistics
Accredited branches: Tokyo, Osaka, Sapporo, Fukuoka

All shipments are coordinated by a transportation professional who has passed Saroute’s training program.
Saroute trains all staff who are involved in the shipping of trial drugs in medical logistics. You can entrust us with everything from answering your questions to transporting your precious cargo.

We Take Care of Packing and Packaging
Our transportation professional will enquire in detail about the nature of your shipment, and select the most suitable packaging. Our transportation staff have also undergone thorough training, so you can leave it up to us to pack your samples as well.

A Range of Fully-Tested Temperature-Controlled Shipping Boxes
All specialized boxes used for temperature control have been tested make sure they meet Saroute’s stringent standards. We can provide transportation containers that maintain a constant internal temperature, coolants (PCM/dry ice) and filled dry vapor shippers.

Convenient PDF Temperature Logs
The use of a temperature data logger to record temperature during transit enables any nonconformity with prescribed temperature to be verified immediately upon receipt. We can also provide you with a log of temperature during transit in PDF format, at a later date.
Examples of Actual Services Provided
-We entered into a contract relating to the transportation of tests drugs and a protocol covering all aspects of the delivery process, including packing, and trained all relevant staff before undertaking transportation.
-We underwent an advance audit and quality check performed by a client and answered questions from the client before commencing service.
Do you need help arranging transport of Biological samples or Investigational products?
Are you facing other logistical challenges in the medical/biotech sector?
Our knowledgeable team of specialists is here to help.
Feel free to ask about our services.
(You can also set up an online meeting to discuss your requirements.)
A Proven Track Record

We delivered Investigational products
directly to patients’ homes.
We can deliver Investigational products stored at hospitals straight to the homes of patients who are unable to travel to the hospital.

Transportation of Clinical Trial Products
Saroute can transport clinical trial products for use in cell therapies.We are also able to temporarily store clinical trial products manufactured overseas in our Japan-based depot.

Integration with Interactive Response Technology
We are able to simplify operational processes by having the interactive response technology (IRT) system used in the clinical trial in question send the request to supply the Investigational product to Saroute directly. We can also negotiate delivery times with the medical institution supplying the drug.
Last Mile Delivery
Process management is important in trial drug logistics, but this is especially true in those cases where Saroute only handles delivery to the medical institution. Saroute is able to integrate with clients’ existing logistics network where necessary.
Do you have questions regarding the transportation of specimens, cells/trial drugs,
DCT logistics or drugs used in clinical trials, or any other medical or biotech challenges?
Are you facing other logistical challenges in the medical/biotech sector?
Our knowledgeable team of specialists is here to help.Feel free to ask about our services.
[You can also set up an online meeting to discuss your requirements.]
Request a Quote
or make an enquiry
Dedicated enquiry hotline 9 am until 6 pm weekdays


