Dedicated enquiry hotline 9 am until 6 pm weekdays
Saroute’s DCT (Decentralized/Distance
Clinical Trial) Transportation Services
TOP > DCT Logistics
Decentralized Clinical Trials (DCTs) make use of online consultations, nurse visits, and wearable devices to eliminate the need for trial subjects to visit medical institutions. DCTs promise to reduce the extent to which a subject’s physical condition and distance to the medical institution creates a barrier to participation, while at the same time boosting enrolment, and reducing the time and cost associated with testing.
What’s DCT Logistics
Learn more about DCT logistics services.
Here we address some common questions regarding decentralized /distance clinical trials.
① What are the requirements associated with the transportation of drugs and medical samples used in these clinical trials
So that trials can be conducted appropriately, drugs and samples need to be properly managed, including with regard to operational arrangements and procedures and temperature control.
② Do we need to change the way we work with visiting nurses
As well as coordinating with the trial institution and accommodating the requirements associated with delivering medical items to the subject’s address, there is also a need to make changes in other areas, including coordination with visiting nurses.
③ How can we establish a robust logistics platform
To ensure the clinical trial runs smoothly, arrangements need to be put in place for the delivery and collection of trial drugs. It is therefore essential to partner with an experienced logistics provider.
④ What issues need to be borne in mind when visiting trial subjects in their homes
When visiting subjects in their homes, service providers need to be mindful of the requested visit time, identification, and the best way of alerting the subject to your arrival.
Saroute will propose the best solution!
Reliable
-All quality control practices employed by Saroute conform to good clinical practice (GCP) and good distribution practice (GDP).
-Our medical logistics professionals, all of whom have completed a specialized training program, will attend to your transportation needs, from start to finish.
Experienced
-Saroute has over 10 years’ experience and expertise in the transportation of clinical trial drugs.
-Saroute prides itself on safe and meticulous processes, and has extensive experience with investigator-instigated trials, large-scale clinical trials, and clinical testing.
Flexible
-We offer temperature-controlled shipping, let you choose the time and mode of delivery, and can otherwise accommodate various requirements.
-We also provide packaging kits and offer an on-site packing service.
Saroute can provide a solution optimized to your requirements,
the size of the trial, and your shipping requirements.
The 2008 amendment to the Japanese Ministerial Ordinance on Good Clinical Practice made it possible for third-party contracts to
transport clinical drugs. Saroute has experience with transporting clinical drugs for both sponsor-initiated and investigator-
initiated trials. We propose optimized solutions tailored to the size of the clinical trial (or remote clinical trial) as well as shipping requirements.
Benefit
1. Access to Standard Agreements and Protocols
The issue: Drawing up agreements and standard operating procedures for decentralized clinical trials is time consuming and progress can be slow.
Benefit of Saroute: By using the standard agreements and protocols prepared by Saroute, you will be able to put in place logistics arrangements more quickly. Arrangements and protocols can also be customized in accordance with trial size and subject.
2. Trouble-Free Delivery to Patients’ Homes
The issue: Some operators do not allow the designation of precise delivery times or do not contact the subject before delivery, meaning delivery can be fraught.
Benefit of Saroute: Our representative will visit at the date and time specified by the trial subject. We can also contact the subject before arrival to reduce the risk of missing them.
3. Trial Subjects Have More Faith in the Delivery Service Thanks to Saroute’s Highly-Trained Staff
The issue: Clinical trials need to be meticulously controlled. We know that clients worry that the person delivering their shipment might not understand what he or she is carrying. Delivery personnel need to possess robust expertise based on specialized knowledge and experience.
Benefits of Saroute: You can rest assured that our staff are fully trained. At Saroute, everyone involved in the delivery process is fully trained. You can count on us to not only manage the delivery of trial drugs and clinical samples, but also to correctly process documentation and reconcile deliveries against documentation.
Case study
Saroute’s DCT (Decentralized/Distance Clinical Trial) Transportation Services are
Used in a Range of Scenarios.
Solution
Saroute draws on its extensive experience with clinical trials to support the transportation of trial drugs and samples for use in DCTs.
Saroute also has extensive DCT experience, and is able to provide standard agreements, protocols, and other documentation suitable for DCTs.
1. Delivery to subjects living near the medical institution
We will arrive at the institution with a temperature-controlled box and deliver the shipment to the patient’s home the same day.
2. Delivery to subjects nationwide
We can deliver trial drugs to patient’s homes nationwide using temperature-control boxes provided by the client. Deliveries are made the same day or the following day.
3. Same-day delivery
We will arrive at the subject’s home with a temperature-controlled box and pack the samples on-site. We will deliver the shipment to the lab on the same day.
Object
Saroute is able to transport
a wide variety of items, including:
- trial drugs
- trial equipment
- blood
- urine
- feces
- sputum
- swabs
- and more
If you want to know whether it is possible to deliver a material not on this list, we would be happy to check the relevant regulations on your behalf.

Service
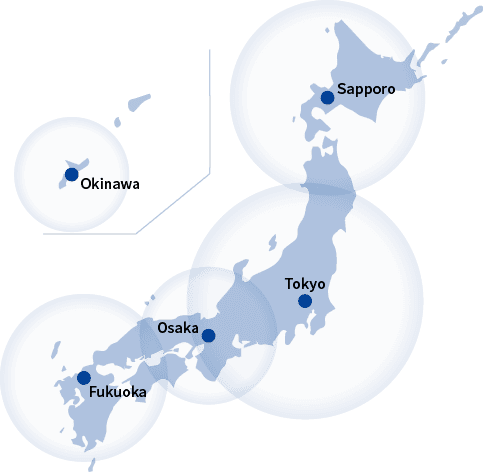
Saroute offers a nationwide service.
In addition to the centers shown on the map, transportation staff who have undergone specialized training are posted in other locations as well. Saroute’s nationwide network extends all the way from Hokkaido to Okinawa.
Saroute’s quality control ensures a high degree of safety and reliability.
Saroute has built a rigorous system of quality controls that govern its medical logistics services. In each part of the network, our professional quality control staff work as a team to ensure safety and peace of mind.
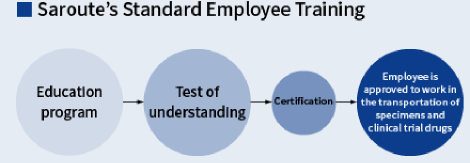
Comprehensive Training for Professional Staff
At Saroute, all staff engaged in providing medical logistics services are required to undergo basic training once a year. Employees are trained in a range of areas including Saroute’s mission, rules in the health sector, Saroute’s standard operating procedure, and compliance. Finally, employees sit a test to check their level of understanding. Individual employees are also trained on procedure and business instructions related to their responsibilities, as necessary.
Whether it’s a one-off order or a large or ongoing project, Saroute provides a service tailored to your needs.
From solutions for the individual specimen transportation requirements of the research community to logistics support for major clinical trials and research conducted at multiple facilities (formulation of transportation networks for clinical specimens), we handle it all. Feel free to contact us with your enquiries.
Our comprehensive service includes everything from transportation
plans to procedure manuals.
Some projects require the assembly of special packaging and the production of procedure manuals. Saroute can provide solutions that fit your requirements, from the design of the overall transportation plan to the formulation and implementation of manuals and protocols.
We Provide Temperature Control at All Ranges
Saroute can provide packaging suitable for various temperatures ranges, including 32 to 38℃, 2 to 8℃, 15 to 25℃, 1 to 30℃, -15 to 25℃,-70℃ or below, -150℃ or below.A temperature data logger can be used to record the temperature of your shipment in transit. We can also provide you with a log of temperature during transit in PDF format.
Expedited/Same-Day Shipping Available Within Japan Specify your
preferred delivery time!
During transit, the conditions of the cargo are monitored. In the event that the shipment is delayed due to unforeseen circumstances, we have alternative means of transport at the ready at all times in order to make up lost time. Our network enables expedited or same-day delivery within Japan. You can also specify the delivery time.
Quotations are free!
We will be able to process your enquiry more easily if you specify the time and date the specimens are to be transported, the details of the shipment (the shape of the cargo, the number of items involved, the nature of the items, and the conditions under which the cargo needs to be transported), and information on the destination.
Do you have questions regarding the transportation of specimens, cells/trial drugs,
DCT logistics or drugs used in clinical trials, or any other medical or biotech challenges?
Are you facing other logistical challenges in the medical/biotech sector?
Our knowledgeable team of specialists is here to help.Feel free to ask about our services.
[You can also set up an online meeting to discuss your requirements.]
Request a Quote
or make an enquiry
Dedicated enquiry hotline 9 am until 6 pm weekdays
